In technological processes of deep purification of natural water from fine suspended and colloidal particles, as well as in wastewater post-treatment, an important place is given to filters with granular loading.
The geometrical structure and physical and chemical properties of the selected filter loading determine the efficiency of the technological process of water conditioning.
According to modern theoretical concepts, the greatest retention capacity has a granular load, with maximum particle surface contacting with water and the smallest hydrodynamic separation force, as well as the largest intergranular and unclosed porosity. On the other hand, the filter media should have increased resistance to mechanical wear in acidic, alkaline and neutral aqueous media.
The vast majority of filter media used in wastewater treatment plants are synthetic. Natural sorbents have been neglected in vain.
Filtering load made of natural zeolite - clinoptilolite:
The natural mineral zeolite belongs to the class of framework aluminosilicates, it is a leader by the totality of useful properties such as sorption, selective ion-exchange, molecular sieve and catalytic properties.Zeolite is a mineral of the clinoptilolite class of aluminosilicates. It is a more effective analogue of quartz sand. The peculiarity of zeolite granules lies in their porosity structure. Zeolite porosity is 16 % higher than that of quartz sand. Zeolite as a cation-exchange mineral has high sorption properties. Zeolite for the water treatment filter as a filling has special thermo- and acid resistance. When using zeolite the filtration cycle is 5 times longer in comparison with quartz sand filling that considerably reduces the costs of water for washing and electricity.
The zeolite granules adsorb a range of different impurities:
- Heavy metals
- Bacteria
- Viruses
- Radioactive elements
- Phenol
- Ammonium
- Nitrates
- Petroleum products
- Organic pollutants
- Ammonia
- Pesticides
- Pathogens
- In addition, zeolite reduces the concentration of chloride ions, fluoride ions, removes hardness salts and softens water.
- Zeolite is particularly effective for the treatment of waste water from nitrogen-containing elements.
- Zeolite has the required mechanical strength. Oxidisability in all media does not exceed permissible norms. (Permissible mechanical strength of filter media for water treatment filters when worn out does not exceed 6.5% when tested by shaking them for 4 hours in a shaking machine).
- Zeolite filters can be used to purify water from copper, manganese, nickel and iron compounds in elevated concentrations. Zeolite as an ion-exchanger of cationic type extracts heavy metals from water, in comparison to synthetic resins possesses high selectivity to cesium and strontium ions.
- Zeolites are also effective against organic compounds; for example, the concentration of benzapyrene, the most common carcinogen in water, is reduced by 60 times.

Natural zeolites are a relatively new class of mineral raw materials used in technological processes of wastewater treatment and post-treatment. The developed specific surface area, good adhesion, adsorption and ion-exchange properties of zeolites make it possible to effectively extract suspended, colloidal and dissolved pollutants of organic and inorganic origin, including ammonium ions, heavy metals and radionuclides from the treated liquid with their help.
Due to the development of adsorption methods for the purification of liquids, there is a growing shortage of adsorbents with high thermal stability and stability in acidic environments.
The acid resistance of natural zeolites makes it possible to use them in such cases in which the use of synthetic zeolites of A and X types is practically impossible. The high mechanical strength of natural minerals makes it possible to eliminate the operation of granulation of the adsorbent. As a result the cost of natural clinoptilolite, which is determined by the costs of extraction, grinding and sieving, is several times lower than the cost of synthetic zeolites.
Advantages of using zeolites in filtration plants
Improvement of the technological parameters of filtration:
The filtration speed for zeolite is 7 - 9 m/hour (5-7 m/hour for quartz),
Filter cycle is increased by 4-16 hours,
Water consumption for washing of the filtering bed decreases by 10-30%,
Filtration rate drop rate is lower;
High sorption capacity of zeolite against algae and microorganisms.
Excessive concentration of ammonia in water is dangerous for pond inhabitants and causes rapid growth of algae. In case of biological pollution, zeolite treatment efficiency is 30-80% higher than quartz treatment;
Provides protection against radionuclides and salts of heavy metals (for decontamination work)
Significant cation exchange, with a feature of high selectivity towards large ions (Rb+, Cs+, Sr++, Hg++). The exchange constants of Na+ ions of clinoptilolite subgroup for Rb+, Cs+, Sr++, Hg++ ions are within 30-63 mg-eq./g.
Zeolite is extremely effective as an adsorbent
In purification of drinking water at sewage treatment plants, water treatment plants in cities, factories, households. Wastewater treatment of cities, companies.
Decontamination of radioactive waste, rubbish, industrial pollution, the elimination of the consequences of environmental disasters.
Cleaning and reclamation of lands, land reclamation, introducing into the soil alone, in composts, prolongation of the action of fertilizers. Increase yields by 0-30%.
Cleaning of polluted water bodies, spawning grounds of small and large rivers from toxic substances and greening.
Filtration of the upper aquifer in water wells.
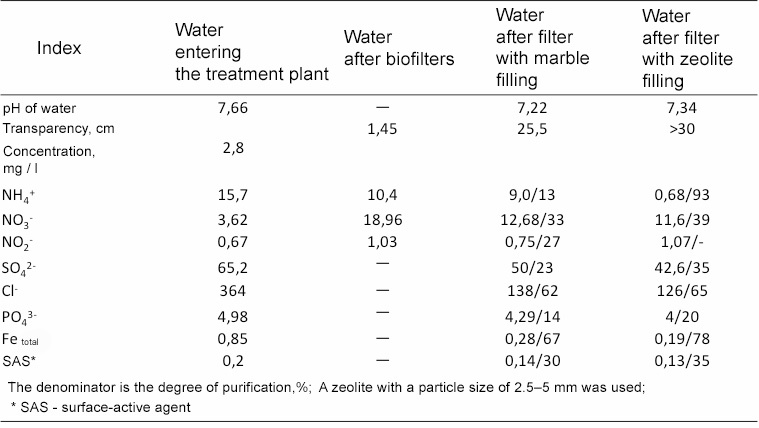
We have studied hydraulic and technological parameters of clinoptilolite filtering loading.
Filtration speed varied within 10-12 m/h, which corresponds to the maximum values of flow speeds in modern rapid filters.
Despite sharp fluctuations in turbidity in the raw water, the quality of the filtrate was stable and within acceptable limits.During downward filtration, a monotonic increase in head loss was observed in the upper loading layers, which is due to saturation of the intergranular pore space with particles of suspended solids of the treated water.
The accumulated sediment in the boot's thickness is periodically (8-24 h) washed with upward flow intensity of 12-14 l/sec.m2. Washing time of silted boot is 5-7 min. Water consumption for sludge removal is 3-4 % of the filter capacity.
The results were compared with the traditional silica sand filter media.
In the whole range of filtering speeds the head loss in the loading layer from clinoptilolite is 1.8-2 times less than in quartz sand, which allows for increasing the efficiency of the water treatment plant at lower energy costs.
The technological process of deep natural water treatment with the use of clinoptilolite filtering loading provides the filtration of biologically treated wastewater through a granular zeolite loading. A two-stage sequential mode of filter operation is recommended. The filtration speed is 6 m/h. The dynamic exchange capacity of zeolite loading is 6.5 mg NH4+/r. To restore the sorption properties of zeolite loading, methods of chemical, biological or joint chemical-biological regeneration have been developed. If it is necessary to extract phosphorus compounds from waste water, a reagent is added to the first stage filter loading.
Wastewater after treatment using this technology can be used in closed systems of technical water supply, as well as discharged into water bodies of I and II categories of water use.
When powdered clinoptilolite, <0.25 mm fraction is used. The cleaning effect is increased by 40-50 % in turbidity, by 1 % in colour and by 40 % in residual aluminium after filtration. In addition, the introduction of clinoptilolite dust reduces the amount of coagulant used by up to 7 %.
During tests, we have studied basic technological parameters of the two-layer zeolite-sand filter (lower layer - quartz sand with a height of 45 cm, upper layer - clinoptilolite sand with a height of 5 cm). The use of clinoptilolitic sand makes it possible to improve physical and chemical indicators of water quality: to reduce turbidity by 6-20% and colour by 5-12%. Zeolite-sandy loading removes iron compounds and products of aluminum sulphate hydrolysis better (by 9-24%).
The study of drying xylene fraction in the liquid phase with natural clinoptilolite zeolite (fraction -2.8 mm) carried out at Kuibyshev Polytechnic Institute showed that clinoptilolite is distinguished by stability of adsorption properties during multicycling and is the most effective adsorbent as compared with traditionally used zeolites (NaA, CaA, NaX).
The total dynamic activity of natural clinoptilolite is about three times higher than that of synthetic zeolites, and the activity before "slippage" is six times higher. The higher efficiency of natural clinoptilolite in relation to adsorption of moisture from xylene fraction is connected with zeolite surface chemistry - the presence of many hydroxyl groups which are centres of physical water adsorption.
The wide range of sorption, from ammonia to cyanides, makes zeolite especially effective in water treatment units of large cities using surface water.
At the same time, the use of zeolite in water treatment plants does not essentially change the technological scheme of the latter.
Zeolite preparation and operation includes: filling zeolite into the filter, soaking, loosening (supply of treated water into the filter from bottom to top and its removal through a tray to the dirty water tank), regeneration (supply of salt solution from the bottom of the salt tank onto the zeolite layer), washing zeolite from common salt (water supply from bottom to top until NH4+ ammonium concentration decreases to 0.9 mg/l).
A 0.1 table salt solution is used as a regeneration agent. The process flowchart provides for the reuse of the salt solution after regeneration followed by ammonia stripping with air.

